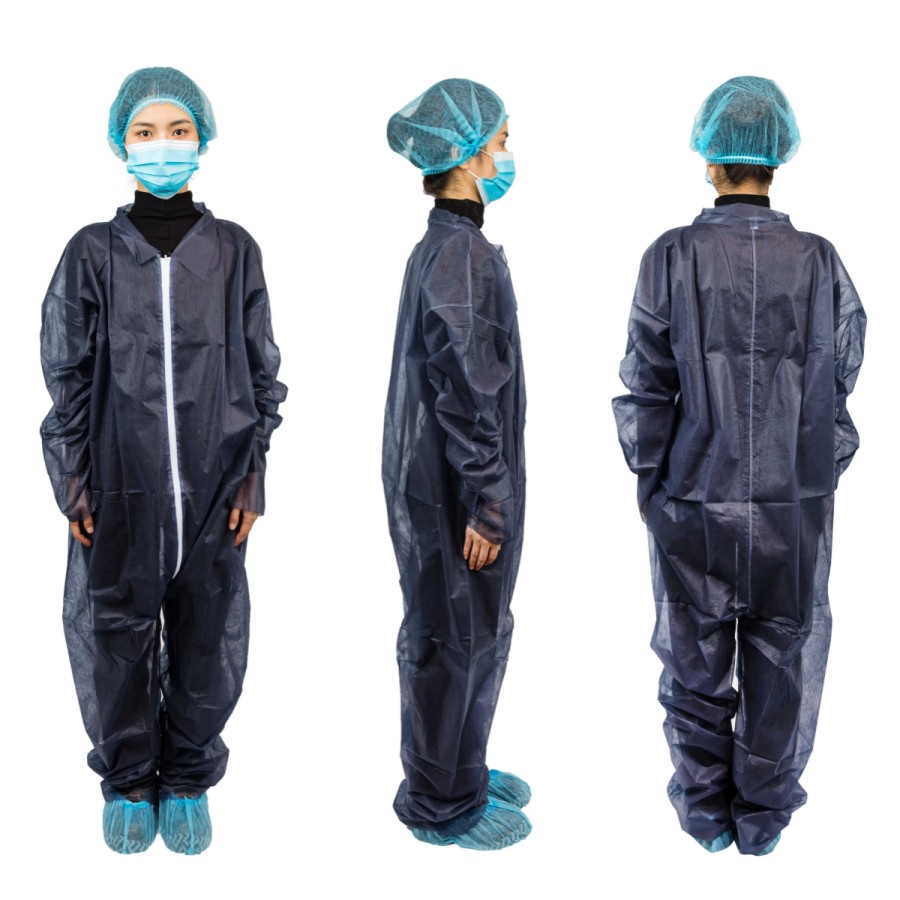
Clean care starts at the top. Hair sheds. Skin flakes. Air moves. In care spaces, small things matter. This is why disposable surgical cap manufacturers play a big role in meeting healthcare compliance. They design, build, and deliver caps that support safe care every day. This guide explains how they do it, what to look for, and how your team can choose well.
What healthcare compliance means for surgical caps
In simple words, healthcare compliance means doing the right thing, the right way, every time. For 일회용 수술용 캡, that includes:
- 위생: block hair and flakes from clean areas
- Fit and comfort: stay in place through long tasks
- Low lint: keep air and tools clear
- Safe materials: gentle on skin, fit for care use
- Clear labels: easy to pick, use, and trace
- Reliable supply: caps ready when shifts start
These goals protect patients and support your team.
How disposable surgical cap manufacturers support compliance
Strong disposable surgical cap manufacturers build systems that hold up under checks and audits. Key steps include:
- Material control
- Pick non woven fabrics like PP, SMS, or spunlace
- Balance breathability, strength, and low lint
- Use skin-friendly elastic and seams
- Design control
- Full hair coverage for short or long hair
- Secure edge at the forehead and nape
- Tie-on options for a custom fit
- Production control
- Clean lines and lot checks
- Regular quality assurance tests
- Clear work rules for staff on the line
- Documentation
- Lot numbers on cases and bags
- Batch records you can trace
- Simple instructions for use
These steps make it easier for your site to show meeting healthcare compliance.
Focus on infection control
A cap is a small barrier with a big job. Good caps help teams:
- Control shedding from hair and scalp
- Reduce droplets from sweat near clean fields
- Lower cross-contamination risk between rooms
- Standardize looks for fast visual checks
For high-risk areas, many sites add full head and side cover. See a close-fit option like a doctor cap with back tie, or pick a full cover like a spunlace surgical hood with ties.
Pick styles that fit real work
Different jobs need different caps. Good disposable surgical cap manufacturers offer clear style choices:
- 부푼 모자 for roomy, soft cover
- Surgical scrub caps with ties for a secure fit
- Clip caps for quick turnover
- Hoods or balaclava caps for full head and neck cover
Explore practical styles:
- A classic blue disposable surgical caps for active work
- A roomy medical bouffant clip cap for nurse and doctor
- A soft low-lint option like a disposable spunlace surgical cap
Material matters
Choose the fabric that matches the task and zone:
- PP 부직포
- Light, breathable, budget smart
- Good for rounds and general care
- SMS
- Strong and low lint
- Solid choice for longer wear
- 스펀레이스
- Very soft and very low lint
- Great comfort for long cases
Your healthcare compliance plan should list where each fabric is used and why.
Labels, sizing, and traceability
Simple labels and smart sizing support clean work:
- Lot numbers on each bag and case
- Sizes that fit many heads and hair types
- Color coding by unit or role
- Clear icons for put-on and take-off steps
Good labels and traceability help during audits and reviews.
Testing and quality assurance
Quality is not a guess. It is proof. Strong disposable surgical cap manufacturers show:
- Fit tests across sizes and hair types
- Lint checks for clean areas
- Elastic strength tests for hold without pain
- Seam checks so edges do not split
- Packaging checks to keep caps clean and dry
This quality assurance helps your team trust each cap, each shift.
Supply chain that meets the moment
A good cap is only good if you have it on time. Look for:
- Reliable lead times and reorder support
- Scalable output for peak seasons
- Clear forecasting with your team
- Backup plans for rush needs
Strong supply helps your site keep healthcare compliance every day.
Training support for your staff
Simple tools help staff use caps the right way:
- One-page guides with pictures
- Short videos on don and doff steps
- Posters at room entries
- Checklists for shift huddles
Training should cover hair tucked, change points, and no re-use.
Eco and waste options
Some sites want to reduce waste while meeting healthcare compliance. Manufacturers can help by:
- Right-sizing caps to avoid extra fabric
- Compact packs to cut storage space
- Clear waste labels for local rules
- Durable yet light designs to lower use per shift
Ask for data that shows use, waste, and savings.
Cost and value
True value mixes cost, quality, and time saved. To find it:
- Compare cost per cap by style and fabric
- Check change rates by unit and shift
- Track waste due to poor fit or tears
- Review delivery times and rush fees
- Balance premium caps in high-risk zones with standard caps elsewhere
Smart mix = strong care + steady budget.
How to choose: quick checklist
Use this list to pick a partner and prove meeting healthcare compliance:
- Does the maker offer all key styles (bouffant, tie-on, hood)?
- Are materials clear (PP, SMS, spunlace) with lint and comfort notes?
- Do they show quality assurance tests?
- Are lot numbers 그리고 batch records easy to trace?
- Is the packaging clean, dry, and easy to open with gloves?
- Are sizes and colors easy to stock and pick?
- Do they support training with simple guides?
- Can they scale and deliver on time?
- Is the pricing fair for the quality you need?
If you can tick these boxes, you have a strong path to healthcare compliance.
Map styles to real rooms
Link the cap to the zone so staff always know what to wear:
- Operating room
- Tie-on scrub caps for most roles
- Hoods in high-splash or high-shedding tasks
- ICU and NICU
- Low-lint bouffant or SMS caps
- Imaging and outpatient
- Light PP caps or clip caps
- Central sterile
- Low-lint options; consider spunlace for comfort
- Visitors
- Easy-on bouffant caps
A posted map makes checks fast and clear.
Fit and comfort tips for staff
Good fit keeps hair in and minds on care:
- Choose the right size
- Tuck all hair, including at the sides and nape
- Use tie-on caps for a custom hold
- Change caps if damp or soiled
- Do not touch the cap during care
For secure all-day wear, see a doctor cap with back tie or pick soft spunlace for long cases: disposable spunlace surgical cap.
Storage and station setup
Keep caps clean, close, and simple to grab:
- Store in a cool, dry place
- Use first in, first out
- Place stations at room entries
- Label bins by style 그리고 크기
- Restock at shift end
You can stock a core set and add special styles as needed:
- Blue disposable surgical caps
- Medical bouffant clip cap for nurse and doctor
- Disposable spunlace surgical cap
- Doctor cap with back tie
- Spunlace surgical hood with ties
Final thoughts
그리고 role of disposable surgical cap manufacturers in meeting healthcare compliance is clear. They plan, test, and deliver caps that help your staff care with confidence. They back each cap with quality assurance, safe materials, clear labels, and on-time supply. Your job is to match styles to zones, train simple steps, and track stock.
Do these things, and your caps will do their job. Hair stays in. Air stays clean. Staff stay focused. Patients stay safe. That is meeting healthcare compliance, shift after shift.