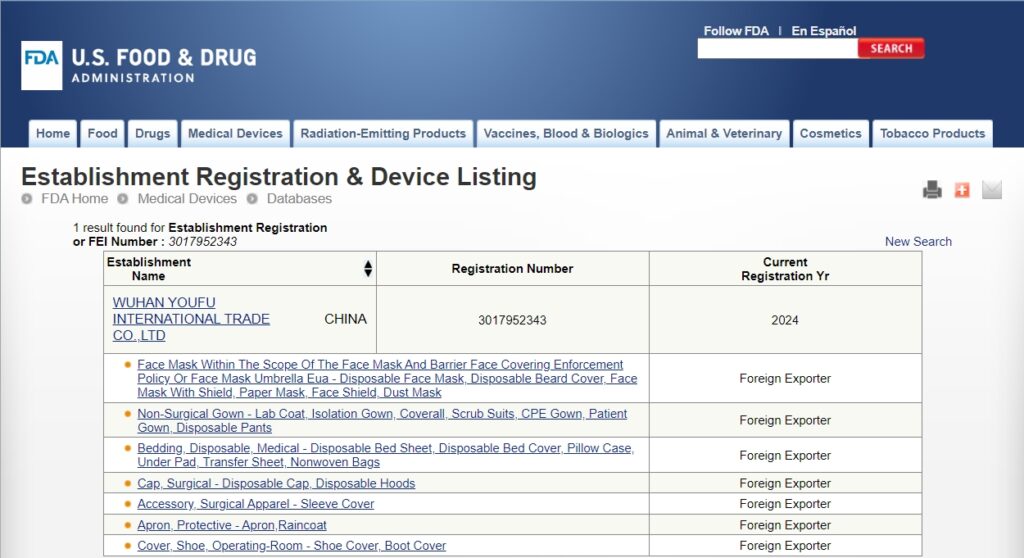
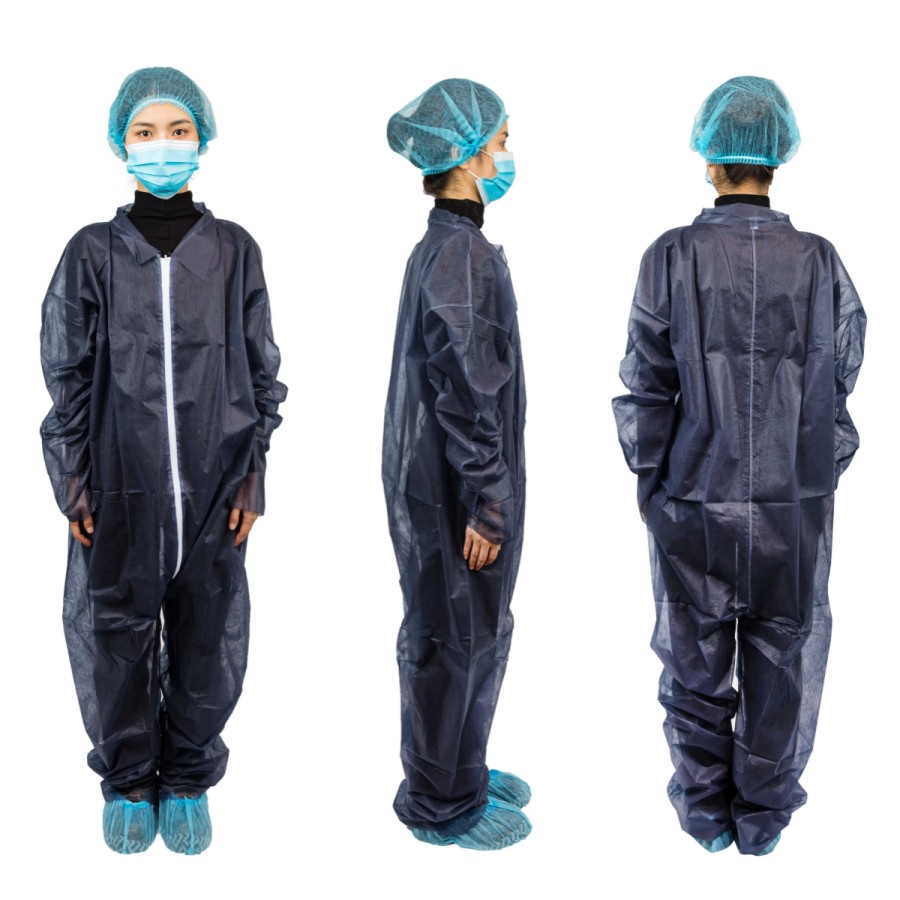
일회용 작업복의 미국 수출에 대한 이해(FDA 규정) starts with clear product facts. Disposable coveralls are one time use suits that cover the body. They can be for general work or for medical use. Your claim sets the rules you must follow. Keep claims simple and true. If you say the suit is for patients or clinics, FDA regulations may apply. If you say it is for general work only, other rules may apply. The safest plan is to match your words to your test data and do not over‑claim.
First, define what you sell and how people will use it. Write short, clear lines that say the who, where및 what. This helps buyers, and it helps border checks. Use plain words, and avoid big promises. Good planning makes the export of disposable coveralls to the USA faster and safer. A simple claim often means a simpler path. A bold claim often needs more proof and may slow your goods at entry. Your team should agree on one claim and keep it the same on all labels and papers.
Next, build a small document pack you can share. The file should show what the suit is, how you make it, and how you check it. Include:
- Product spec: fabric, weight, size range, color.
- Parts list: zipper or flap, elastic, seam type, tape if used.
- Process flow: main steps from cut to pack.
- QC plan: seam checks, size checks, zipper pull, visual check.
- Test notes: skin contact safety, seam strength, water spray, basic flame behavior.
- Labels and use steps: artwork, warnings, and 1회 사용 note.
- Lot trace: code system and how you track each batch. Keep versions and dates clear. Store files in one place so you can answer fast when asked.
Good labels and clean entry data are key. Your pack label should show:
- Product name, model, and one time use notice.
- 크기, material, lot number및 date mark.
- Maker name, contact, and country of origin.
- Short use steps and simple warnings. If you claim medical use, set up the needed steps with FDA (such as registration and listing). At the border, your entry must match your invoice, pack list, and case labels. Use the right tariff code. Seal cartons well, and keep marks clear so teams can scan and move them with no delay.
Keep steady quality and plan your time. Use the same fabric weight and color by lot. Test seams and zippers often. Check sizes by lot. Store goods clean and dry. Set a simple timeline and stick to it:
- Choose fabric, write claim, lock specs.
- Run tests, make labels, set FDA steps if needed.
- Make a pilot lot, check QC, and fix issues.
- Book freight, prepare entry, and ship. Avoid common mistakes: do not over‑claim, do not ship with weak labels, do not change specs without notes, and do not ship without test proof. With clear claims, clean labels, and steady data, 일회용 작업복의 미국 수출에 대한 이해(FDA 규정) becomes a clear, safe path from your line to your buyer.