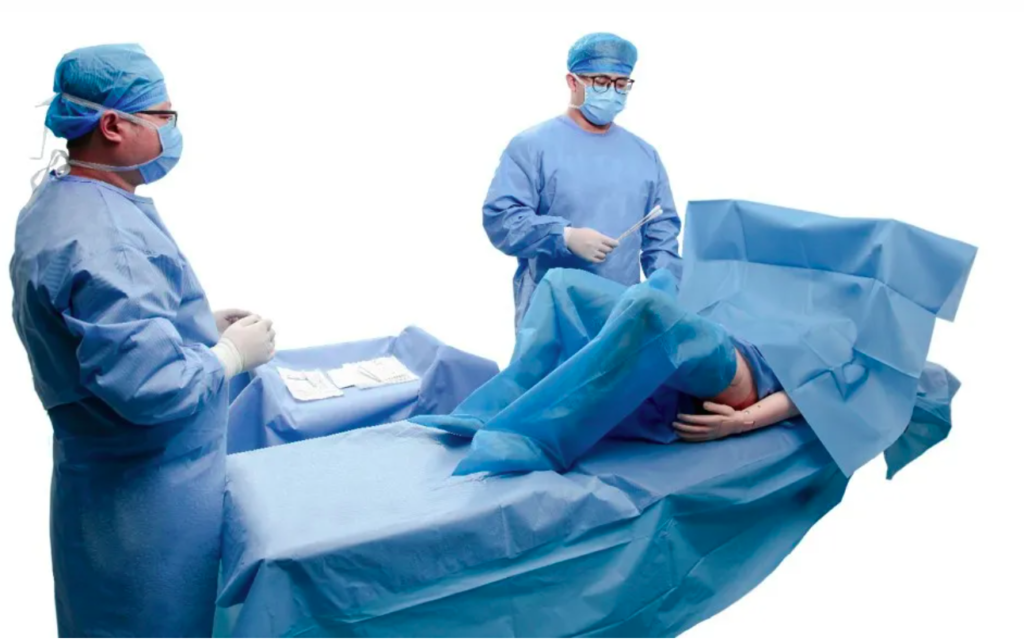
A disposable delivery pack is a single‑use set made for birth care and clean work. It helps set a sterile field fast. A pack can hold many basic items, and all items are packed together. Common parts may include drapes, gowns, towels, gauze, swabs, wraps, and small tools. The intended use and the parts inside guide the class of medical device for the pack. In many places, a delivery pack is seen as a procedure pack. That means the class can change with the risk of the items inside the pack.
The medical device classification is based on how the pack is used and how it touches the patient. The key idea is risk. Low risk items lead to a lower class. Added risk leads to a higher class. Think about these points when you set the class:
- Intended use: labor, birth, or general clean care.
- Contact: intact skin only, or blood and tissue contact.
- Sterility: sterile or non‑sterile pack.
- Active parts: no powered items vs. powered or sharp items.
- Time of use: short, single task vs. longer contact.
Many disposable delivery packs with basic non‑active parts are usually a lower class. They are single‑use and sterile, and they help keep a clean field. Examples of lower risk parts include:
- Drapes and gowns for barrier use.
- Towels, gauze, and swabs for clean up.
- Wraps and trays that hold items in place. If the delivery pack adds higher risk parts, the class can rise. Examples of higher risk parts include:
- Sharps like a scalpel blade or needle.
- Devices that go inside the body.
- Active devices that use power. The more risk in the pack, the higher the likely class.
Your labeling and packaging must match the class of medical device and the intended use. Clear labels help users pick the right pack and use it right. Good labels and papers often show:
- Product name, procedure pack note, and single‑use mark.
- Sterile mark (if sterile) and the sterilization method.
- Contents list and sizes.
- Lot number, manufacturing date, and expiry date.
- Instructions for use, simple warnings, and storage notes. Strong packaging keeps the sterile barrier safe to the shelf life date. Do not sell or use if the wrap is open or torn.
Makers should build a clean file that supports the class and claim of the disposable delivery pack. Keep the claim simple and true. Link the claim to your tests and checks. A basic plan can include:
- Defined intended use and user group.
- A full components list and drawings.
- Quality control checks and validation of key steps.
- Sterilization method and seal strength checks (for sterile packs).
- Traceability from raw parts to final lot number. Buyers should match the intended use at the site to the claim on the pack. Pick sterile for a sterile field. Pick non‑sterile only for tasks that do not need a sterile field. When the claim, the risk, and the parts all line up, the class of medical device for disposable delivery packs is clear and fit for safe care.