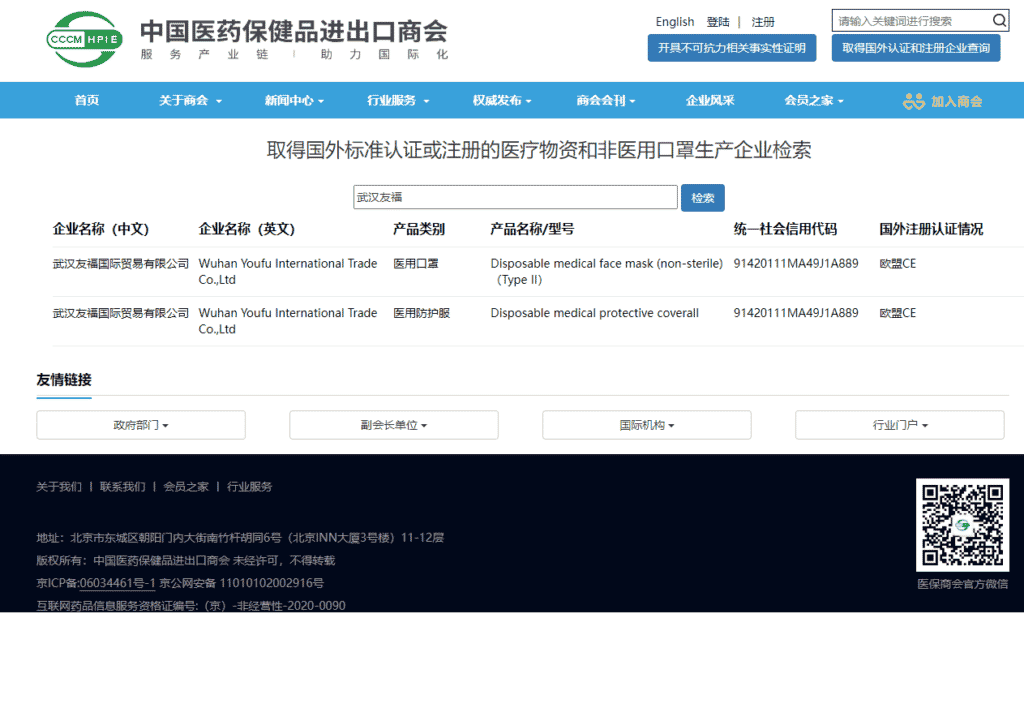
Wuhan Youfu International Trade Co., Ltd. officially entered the “white list” of the Ministry of Commerce for the export of medical devices, and became a “white list” enterprise of the Ministry of Commerce. Since then, our company’s disposable medical face mask(non-sterile)(TYPE II) and disposable medical protective coverall have been exported to the international market with a customs clearance “talisman”, and the company’s production capacity has reached 1 million masks and 20,000 protective clothing per day. Liu Yu, our CEO said: “The ‘white list’ is an affirmation of the enterprise and the driving force for the development of the enterprise. In the next step, we will further expand the scale and consolidate and expand both domestic and foreign markets.”
On April 25, 2020, China’s Ministry of Commerce (MOFCOM), the General Administration of Customs (GACC), and the State Administration for Market Regulation (SAMR) jointly issued the Announcement NO.12 of 2020 of Ministry of Commerce, General Administration of Customs and State Administration for Market Regulation on Reinforcing Quality Regulation on Exported Supplies for COVID-19 Response. This statement revised a previous policy that allows only National Medical Product Administration (NMPA, or commonly known as CFDA) certified medical equipment to export. GACC will inspect and release the exports according to a list of manufacturers with foreign standard certification or registration provided by MOFCOM, which is known as China Medical Device Exportation Whitelist.
Wuhan Youfu International Trade Co., Ltd. is a modern medical device production and sales enterprise integrating R&D, production and sales. It mainly produces and sells disposable medical masks and disposable civilian masks, medical protective coverall and regular protective coverall for daily protection. Under the guidance and declaration of commercial departments at all levels, Wuhan YOUFU’s disposable coveralls and disposable face masks have both obtained CE certification, and are both listed on the MOFCOM medical device exportation whitelist.